Reports
Optimized Perfusion by Capacitance Process Measurement & Control
Recent studies on biotherapeutic protein production have shown perfusion processes as a superior technology vs. the traditional batch and fed batch approaches. Due to its associated process stability and reducing effect of varying conditions inside the bioreactor, Perfusion can deliver lower production costs and higher titer, especially in the case of low titer or fragile proteins.
As the focus on in-line techniques for bioprocess monitoring is increasing, driven primarily by the PAT / FDA initiatives, one of the most important, yet also the most challenging components to monitor on-line and real time is biomass, a critical process parameter that significantly impacts the quality attributes of the process/product.
Among all the available on-line biomass assays, the capacitance method has a clear advantage for process development and manufacturing because it is an unambiguous reflection of viable cell biovolume rather than the total number of cells (Carvell & Dowd, 2006). The viable cell concentration is of prime importance in metabolic studies and those relating to the efficiency of target protein production.
This application note explores the benefits of utilizing the FUTURA capacitance technology to perfect the online measurement and control of viable cell density which is essential for an optimal Perfusion run.
Principle of ABER’s Cell Capacitance Technology
Cells with intact plasma membranes can be considered to act as tiny capacitors under the influence of an alternating electric field. The non-conducting nature of the plasma membrane allows a build-up of charge and this is measured as capacitance in (pF/cm) which can be converted to a relatable unit such as cells/ml or g/L. The capacitance measured is dependent upon cell type and is directly proportional to the membrane bound bio volume of viable cells. FUTURA also measures the Conductivity of the medium, in milli siemens per centimeter (mS/cm). Conductivity is not used to measure biomass but is indicative of the production or utilization of ions by the cell suspension.
Optimized Perfusion Process Monitoring and Control
Accurate control of the feed or addition rates are an Essential Requirement in Perfusion Processes. Maintaining pseudo-steady-state conditions in these bioreactors can be especially challenging due to high and fluctuating cell concentrations that can rapidly change environmental conditions. With infrequent, manual viable cell counts based on trypan blue exclusion, the control system can have too little information on which to base an appropriate decision to manipulate the process, and hence will lead to large process deviations. By introducing tight control of the perfusion or concentrate addition rate allows the bioreactor to be operated under the optimum conditions for maximum recombinant protein production.
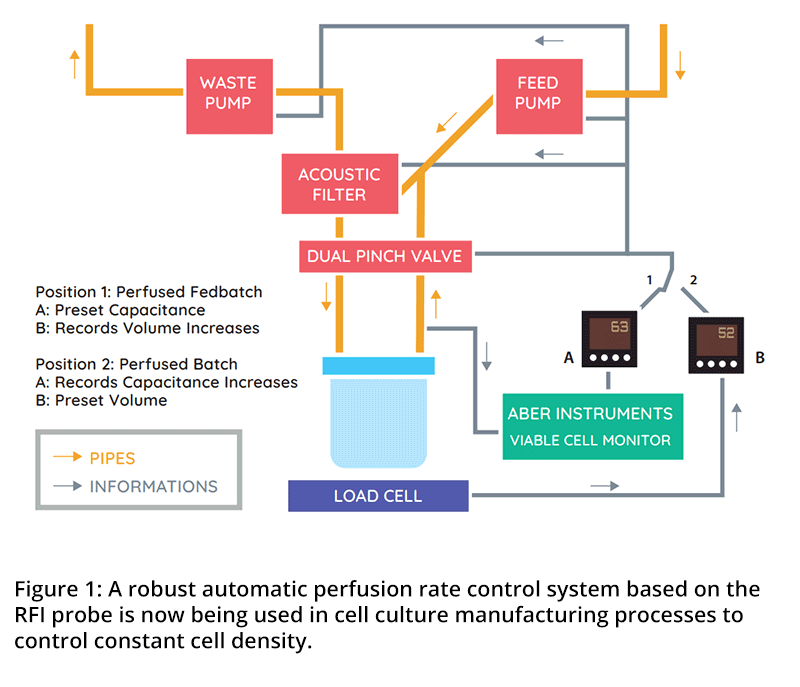
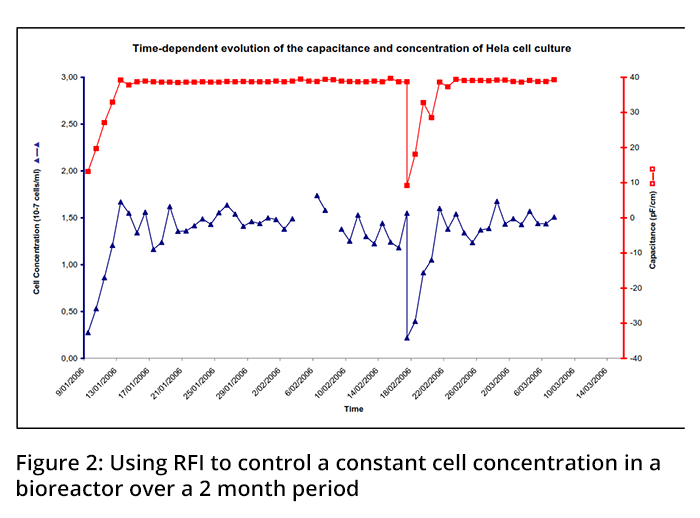
A robust automatic perfusion rate control system based on the Aber capacitance probe has been demonstrated in continuous cell culture manufacturing processes. The system operates in a completely closed loop i.e. no samples need to be taken to obtain process information. In the control algorithm, a cell specific perfusion rate is specified and the capacitance signal is converted into a perfusion flow rate through calculation and implementation with a variable speed-controlled pump (Figure #1). An example of a time-dependent capacitance trace of a perfused Hela cell culture evolving from batch (preset volume, increasing concentration) to fed batch (increasing volume, preset cell concentration) growth conditions in shown in Figure #2. The stable capacitance value can be seen when the culture is operated in a Fedbatch mode with a preset cell concentration of 107 cells/ml. The peak observed during this step represents an increased cell concentration due to an insufficient fresh medium supply to maintain a stable capacitance.
The capacitance probe only measures the viable cell mass and is therefore ideal for this application and it has been applied for process control in sono-perfused cytostats, spin-filter perfused bioreactors and for maintaining steady-state, continuous culture of bioreactors with external loop filters (eg the Repligen ATF system) for monoclonal antibody and recombinant protein production.
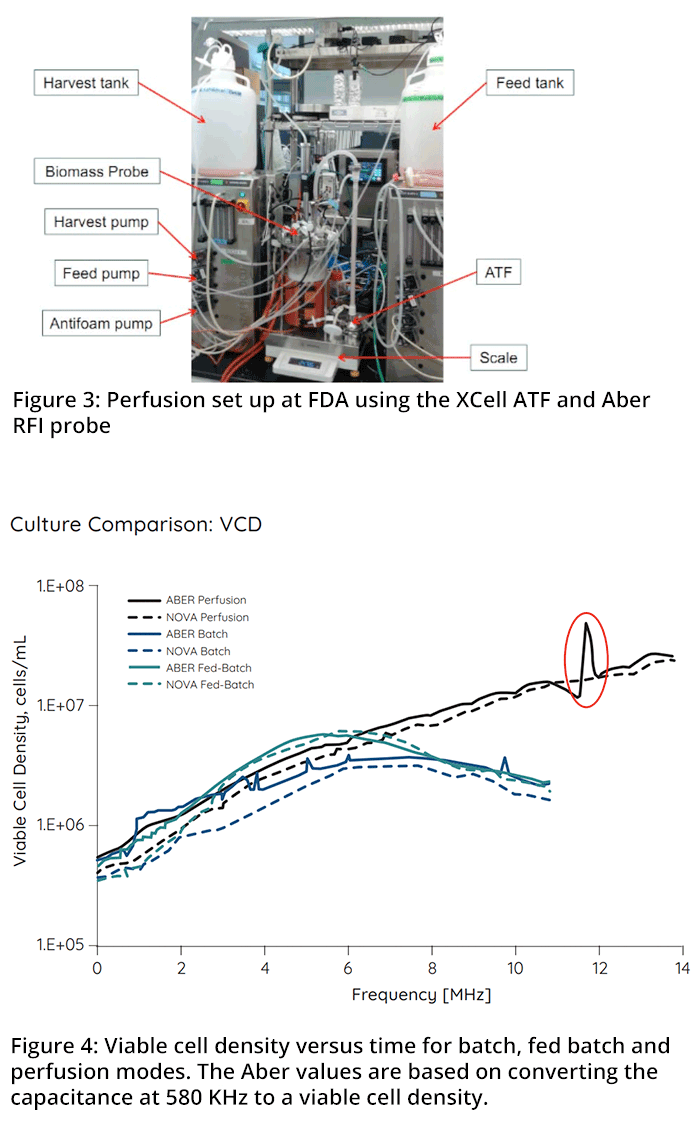
In the second example of process control, data are taken from the FDA (Division of Biotechnology Review and Research-II OBP/OPQ/CDER). 5-L glass bioreactors were run in batch, fed batch and perfusion modes. In the perfusion mode the bioreactor was equipped with an XCell ATF device (Repligen) and a capacitance probe. A photograph of the experimental set up is shown in Figure #3.
In Figure #4, the viable cell densities for cultures run in the three different modes are compared between the Nova Bioprofile method (based on image analysis and Trypan blue) and the Aber values. The Aber FUTURA VCD is based on converting the capacitance at 580 KHz to a viable cell density using a linear model. The capacitance data correlated well with the Nova Bioprofile VCD in both the fedbatch and perfusion modes. In the batch mode, the data were skewed to match the VCD during the death phase leading to a slight overestimation during the growth phase. The discrepancies that sometimes occur between the capacitance and trypan blue methods to derive the VCD during the cell death phase are now well understood and are explained in the literature (Braasch et al, 2013, Lee et al, 2014). In perfusion mode, a sudden increase in capacitance demonstrated the ability to immediately spot a sudden increase in live cell density after 12 days of culture caused by too much media being pumped out in error. This highlights an additional advantage of using a capacitance probe to measure the VCD in real time so that errors or sudden failures can be picked up and that appropriate corrective action can be taken.
Learn more about Aber’s FUTURA products available from PROAnalytics
References in Continuous/ Perfusion Cell Culture and Process Control
Carvell J, Bhat A, Jones R. (2017) Using Radio-Frequency Impedance to Control Continuous High Density Perfusion Culture with the Alternating Tangential Flow System. Poster Presented at ESACT 2017, Switzerland (in press).
Dowd JE, Jubb A, Kwok KE, Piret JM (2003) Optimization and control of perfusion cultures using a viable cell probe and cell specific perfusion rates. Cytotechnology 42:35–45.
Justice C, Brix A, Freimark D, et al (2011) Process control in cell culture technology using dielectric spectroscopy. Biotechnol Adv 29:391–401.
Karst, D.J., Steinebach, F., Soos, M. and Morbidelli, M., 2017. Process performance and product quality in an integrated continuous antibody production process. Biotechnology and bioengineering, 114(2), pp.298-307.
Sergeant D, Moser M, Carvell JP (2007) Measurement and control of viable cell density in a mammalian cell bioprocessing facility: Validation of the software. 20th ESACT meeting Dresden.
Sergeant et al (2006) Controlling a constant level of viable biomass in a perfused cytostat using radio-frequency impedance. Poster presented at Cell Culture Engineering Conference X, April, 2006.
Nanjegowda et al (2013) Applications of biomass probe in PAT. Poster presented at 23rd ESACT Meeting Lille, France: Published in BMC Proceedings (Supplement 6): 89.
Zhang A, Tsang VL, Moore B, Shen V, Huang YM, Kshirsagar R, Ryll, T. (2015) Advanced Process Monitoring and Feedback Control to Enhance Cell Culture Process Production and Robustness. Biotechnol and Bioeng; 9999: 1-1.